dupilumab conjunctivitis treatment
The present review highlights the clinical presentation of dupilumab-associated conjunctivitis and addresses pharmacological and non-pharmacological options available for the treatment of this clinically highly relevant condition. Most patients with conjunctivitis dupilumab 1217.
Dupilumab Zur Behandlung Der Therapierefraktaren Atopischen Dermatitis Springerlink
Up to 1 in 5 people taking dupilumab develop conjunctivitis which can cause itching redness and dryness of the eye.
. Most conjunctivitis events were mild to moderate. In PED-OLE 12275 adolescents 44 reported one or more conjunctivitis event. 1 a b in patients with AD with conjunctivitis vs.
75 of patients 9 of 12 including the three patients who discontinued treatment had an Investigator Global Assessment IGA score of 4 severe before starting the drug. Dupilumab may cause conjunctivitis. In PED-OLE 12275 adolescents 44 reported one or more conjunctivitis event.
Placebo 44 recoveredresolved during the treatment period. Mean age of dupilumab-treated patients was 36 years range 18 to 83 years and 785 were men. National guidelines recommend dupilumab for the treatment of moderate to severe eczema in adults who have not improved with at least one tablet medication.
Two patients permanently discontinued dupilumab due to conjunctivitis or keratitis. Objective To perform a bibliometric analysis of all the qualified literature involving ocular AEs during the treatment of AD with dupilumab. Most cases were diagnosed by the investigators.
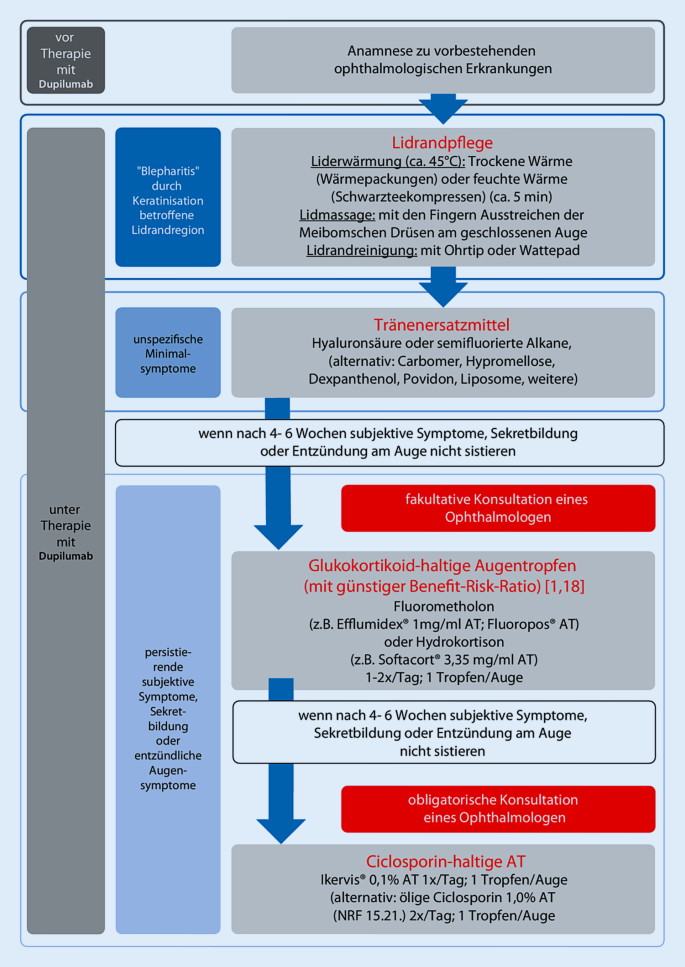
Dupilumab the first targeted biological drug approved for the treatment of AD has been widely used along with increasing ocular adverse effects AEs. Topical tacrolimus eyelid oin tment. Conjunctivitis was mostly mild to moderate.
Glaucoma cataracts posterior subscapular and and infectious keratoconjunctivitis may complicate therapy with corticosteroids. Most conjunctivitis events were mild to moderate. Placebo 44 recoveredresolved during the treatment period.
Mean age of patients with conjunctivitis was 35 years range 18 to 53 years. The most prominent histopathological feature in conjunctival biopsies from patients with AD developing conjunctivitis during dupilumab treatment was a scarcity of intraepithelial GCs. Most patients with conjunctivitis dupilumab 1217.
Further studies are needed to support the use of this drug for dupilumab-related conjunctivitis. Most cases recovered or resolved during the treatment period. Common treatments included ophthalmic corticosteroids antibiotics and antihistamines or mast cell stabilizers.
Referral to an ophthalmologist. Mean EASI score of patients with conjunctivitis was 37 range 26 to 60 which was higher than the mean EASI score of the total N277 cohort. Most patients with conjunctivitis dupilumab 1217.
Dupilumab is the first biologic approved for use in treatment of moderate to severe atopic dermatitis AD 1 2 3 4 5. In PED-OLE 12275 adolescents 44 reported one or more conjunctivitis event. Overall 25 percent of patients 3 of 12 developed severe enough conjunctivitis that dupilumab treatment was discontinued.
The risk of conjunctivitis showed no relationship with dupilumab serum concentration. Mild conjunctivitis is managed with lubricating eye drops. The conjunctivitis occurred at a mean of 158 weeks of treatment range 8-41 weeks and was considered severe or moderate-to-severe in 4 patients.
The risk of conjunctivitis showed no relationship with dupilumab serum concentration. Conjunctivitis a predisposition to keratoconus and anterior subcapsular cataracts are considered minor criteria of AD. This case was remarkable as the medication applied externally to the eyelid skin was effective in treating the conjunctival involvement possibly due to penetration of pimecrolimus through the eyelid layers.
Dupilumab a monoclonal antibody that inhibits both interleukin IL-4 and IL-13 signaling is an effective treatment option in moderate-to-severe atopic dermatitis AD. 283 and 363 cells mm 1 in the two control samples. Key clinical point.
Median GC density was 33 cells mm 1 IQR 1149 Fig. Treatment options include topical corticosteroids and topical calcineurin inhibitors. Those who initiated other systemic therapies with comorbid asthma further increasing the risk.
Patients with atopic dermatitis AD who initiated dupilumab were at a 2-fold higher risk of developing conjunctivitis within 6 months of treatment initiation vs. In the dupilumab group the conjunctivitis was classified as mild 22 56 of 39 or moderate 17 44 of 39 in intensity. Dupilumab targets interleukin IL-4.
Patients on dupilumab should be advised to use these from the onset of treatment to prevent ocular symptoms. Most conjunctivitis events were mild to moderate. At baseline 9 75 of the 12 patients had severe AD.
The mean age at conjunctivitis onset was 30 years. Some patients may require other treatment. Placebo 44 recoveredresolved during the treatment period.
The risk of conjunctivitis showed no relationship with dupilumab serum concentration. Dupilumab was stopped in 3 patients all of whom had severe conjunctivitis. Patients with AD are already at increased risk of developing conjunctivitis.
One patient in the dupilumab group discontinued the study because of mild allergic conjunctivitis that started on study day 29 and was considered possibly related to study treatment.

Moderate Conjunctivitis And Blepharitis At Week 22 Of Dupilumab Treatment Download Scientific Diagram
Conjunctival Cicatrisation In Patient On Dupilumab Patient On Download Scientific Diagram

Dupixent Dupilumab Mechanism Of Action

Long Term Follow Up And Treatment Outcomes Of Conjunctivitis During Dupilumab Treatment In Patients With Moderate To Severe Atopic Dermatitis The Journal Of Allergy And Clinical Immunology In Practice

Dupilumab Associated Ocular Surface Disease Incidence Management And Long Term Sequelae Medrxiv

Dupilumab Senkt Den Juckreiz Und Hebt Die Lebensqualitat Rosenfluh Ch
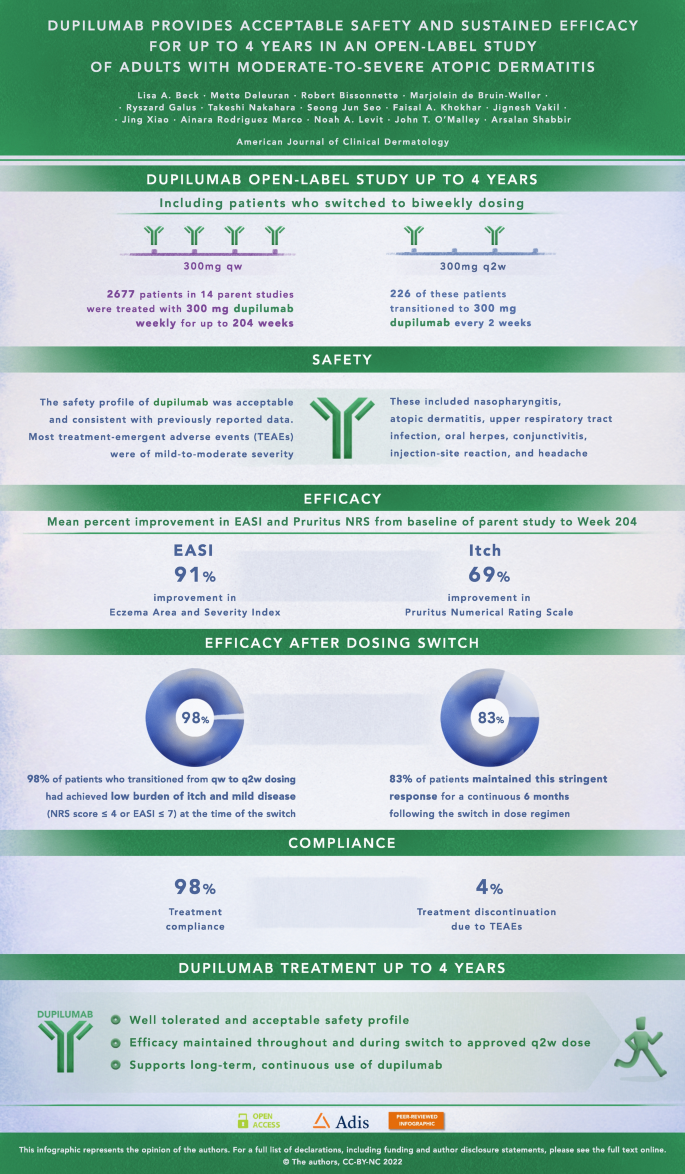
Dupilumab Provides Acceptable Safety And Sustained Efficacy For Up To 4 Years In An Open Label Study Of Adults With Moderate To Severe Atopic Dermatitis Springerlink

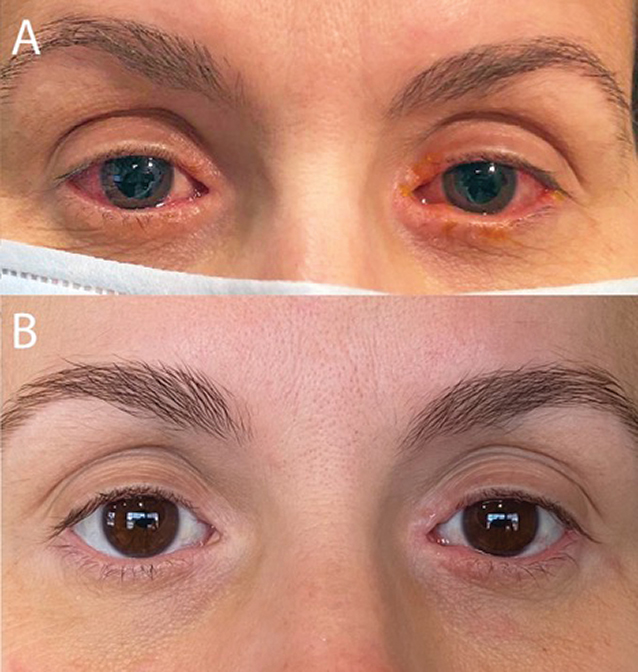
Example Of Case Before And After Treatment With Dupilumab 300mg Every Download Scientific Diagram

Dupilumab Associated Ocular Surface Disease Incidence Management And Long Term Sequelae Medrxiv

Pin By Ian Palmer On Magazine Ad Atopic Dermatitis Lactation Hypersensitivity

Clinical Findings Of Bilateral Blepharitis And Conjunctivitis After Download Scientific Diagram

Dupilumab An Opportunity To Unravel In Vivo Actions Of Il 4 And Il 13 In Humans Sciencedirect

A A Man Aged 46 Years 6 Weeks After Starting Dupilumab Marked Download Scientific Diagram

New Insights On Ocular Surface Disease In Patients With Atopic Dermatitis Treated With Dupilumab Bortoluzzi 2022 British Journal Of Dermatology Wiley Online Library

Real World Persistence With Dupilumab Among Adults With Atopic Dermatitis Annals Of Allergy Asthma Immunology

Dupilumab Sar231893 For Eczema Infantile Clinical Trial 2022 Power

Efficacy Of Dupilumab In Atopic Comorbidities Associated With Moderate To Severe Adult Atopic Dermatitis Nettis 2020 Allergy Wiley Online Library

Clinical Improvement Of Patients After 16 Weeks Of Dupilumab Treatment Download Scientific Diagram
Comments
Post a Comment